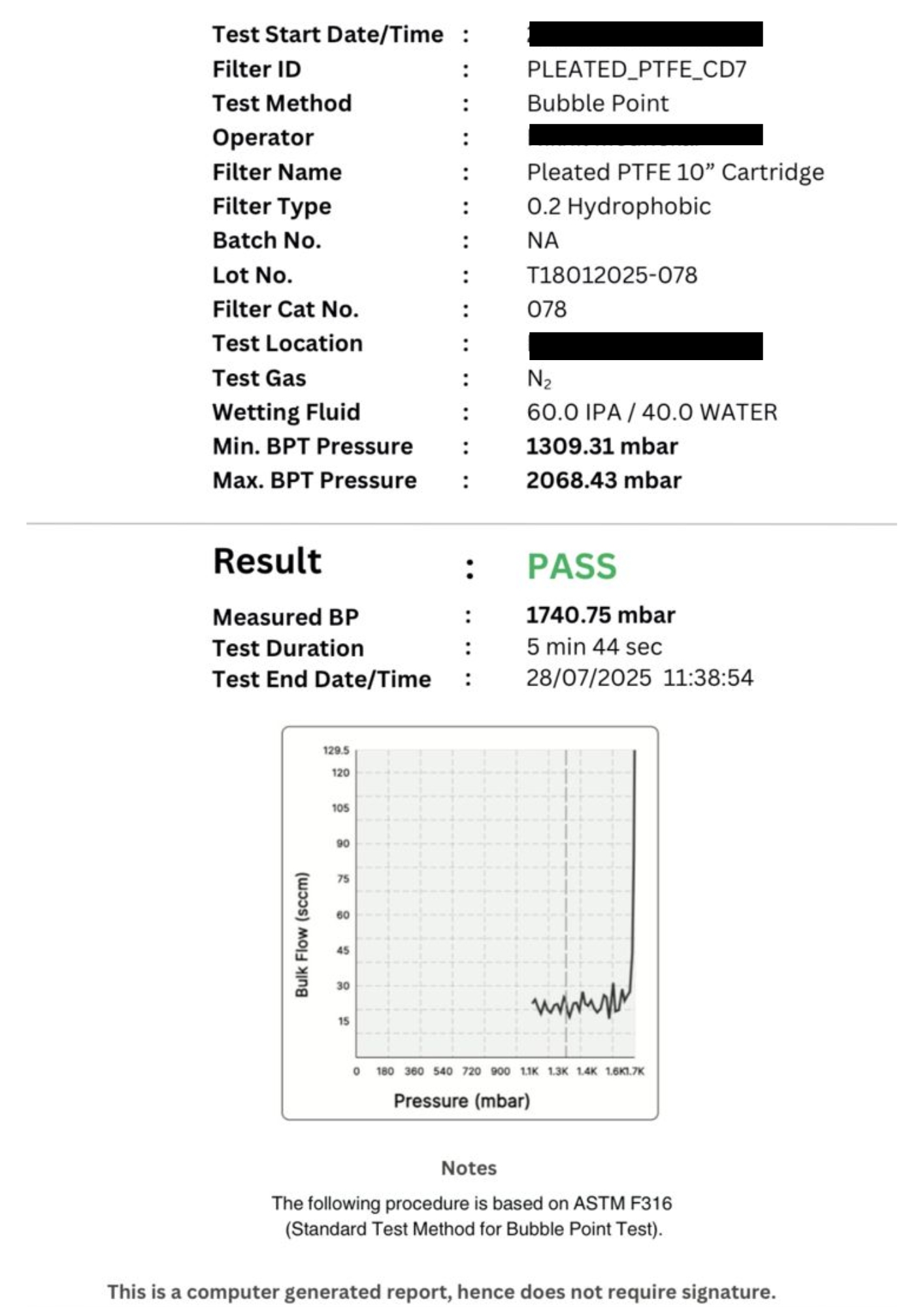
-
Nondestructive test (filter remains usable after testing)
-
Rapid, reliable, and repeatable
-
Detects oversized pores or defects in membranes
-
Correlates with filter pore size and retention rating
-
Widely accepted in regulatory guidelines (FDA, EMA, WHO, ISO)
-
Suitable for in-line or offline validation setups
-
Filter Types: Hydrophilic and hydrophobic membranes (with pre-wetting for hydrophobic)
-
Configurations: Single cartridge, capsule filters, disc filters
-
Liquids for Wetting: Water, isopropanol-water mixture (for hydrophobic membranes)
-
Test Equipment: Manual integrity testers, automated digital integrity testers
-
Documentation: 21 CFR Part 11 compliant electronic records (for pharma validation)
-
Typical Test Pressure Range: 200 mbar to 7 bar (depending on filter rating)
-
Filter Pore Size Correlation:
-
0.1 µm → ~3.5 bar
-
0.2 µm → ~2.4 bar
-
0.45 µm → ~1.0 bar
-
0.65 µm → ~0.6 bar
-
-
Gas Used: Compressed air or nitrogen
-
Accuracy: ±2% of full scale (automated systems)
-
Compliance: ASTM F316, PDA TR26, ISO 29463, EN 1822, GMP Annex 1
-
Sterile filtration validation in pharma manufacturing
-
Biotech fermentation media and buffer filtration
-
Water-for-Injection (WFI) and purified water systems
-
Final filtration in food & beverage (beer, wine, soft drinks)
-
Microelectronics ultrapure water systems
-
Medical device manufacturing sterility assurance